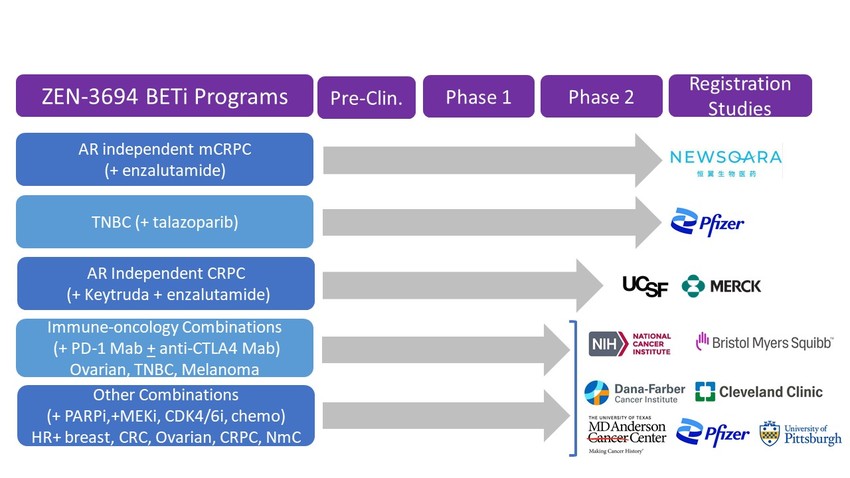
Our development strategy is focused on targeting patient subsets with high unmet need and those that are most likely to benefit from therapy. We are advancing multiple clinical programs targeting BET bromodomains in combination with other drugs in oncology indications.
Our lead product, ZEN-3694, a BET inhibitor, in combination with enzalutamide, is in a Phase 2 randomized clinical trial for treatment of metastatic castration resistant prostate cancer (mCRPC) in patients that have progressed on an androgen receptor signaling inhibitor.
Also, in collaboration with Pfizer, we are advancing ZEN-3694 in combination with talazoparib in a Phase 2 study in triple negative breast cancer (TNBC) patients that do not have a hereditary BRCA1/2 mutation. This is expected to greatly expand the patient population currently addressed by PARP inhibitors.
Clinical trials combining ZEN-3694 with immune check point inhibitors and kinase inhibitors are being initiated.