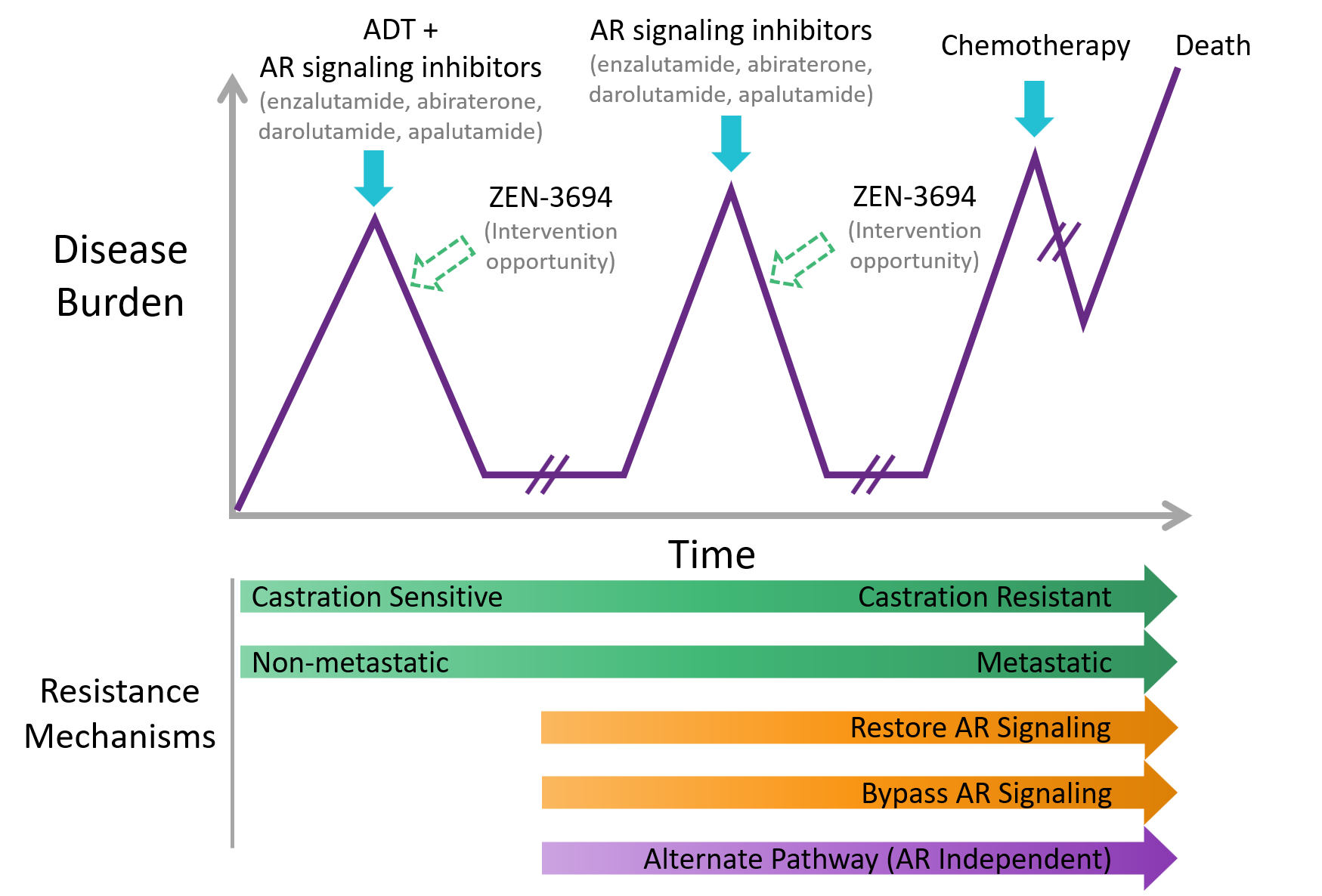
We conducted a first-in-human Phase 1b/2 dose escalation/expansion study of ZEN-3694 in combination with enzalutamide in patients with metastatic castration resistant prostate cancer (mCRPC) and prior progression on one or more androgen signaling inhibitor. Results demonstrated that ZEN-3694 has acceptable tolerability and encouraging preliminary efficacy data in combination with enzalutamide in patients with mCRPC. The median radiographic progression-free survival (rPFS) in the overall cohort was 9 months.
The efficacy was particularly impressive in the subset of patients that had previously responded poorly to abiraterone and were more likely to be androgen receptor (AR) independent. These AR independent patients are expected to have a poor response to enzalutamide – a current standard of care therapy, because the tumor is insensitive to inhibiting AR. The clinical and pharmacodynamic data suggests that the combination of ZEN-3694 and enzalutamide may be able to thwart resistance mechanisms and re-sensitize patients to AR-signaling inhibitors.
Epigenetic Therapeutic Targets Summit 2020 Presentation
Prostate cancer is the most common malignancy and second leading cause of death among men in the United States. Multiple resistance mechanisms have been identified which hinder current standard of care therapeutics from effectively combatting the disease. ZEN-3694 is an orally bioavailable, second generation, potent pan-BET bromodomain inhibitor that has significant preclinical efficacy as single agents, with evidence of synergy when combined with enzalutamide.
An expanded Phase 2b randomized trial is currently being planned which will test ZEN-3694 + enzalutamide vs enzalutamide in randomized patients that have previously responded poorly to abiraterone.
Disease Progression in Prostate Cancer